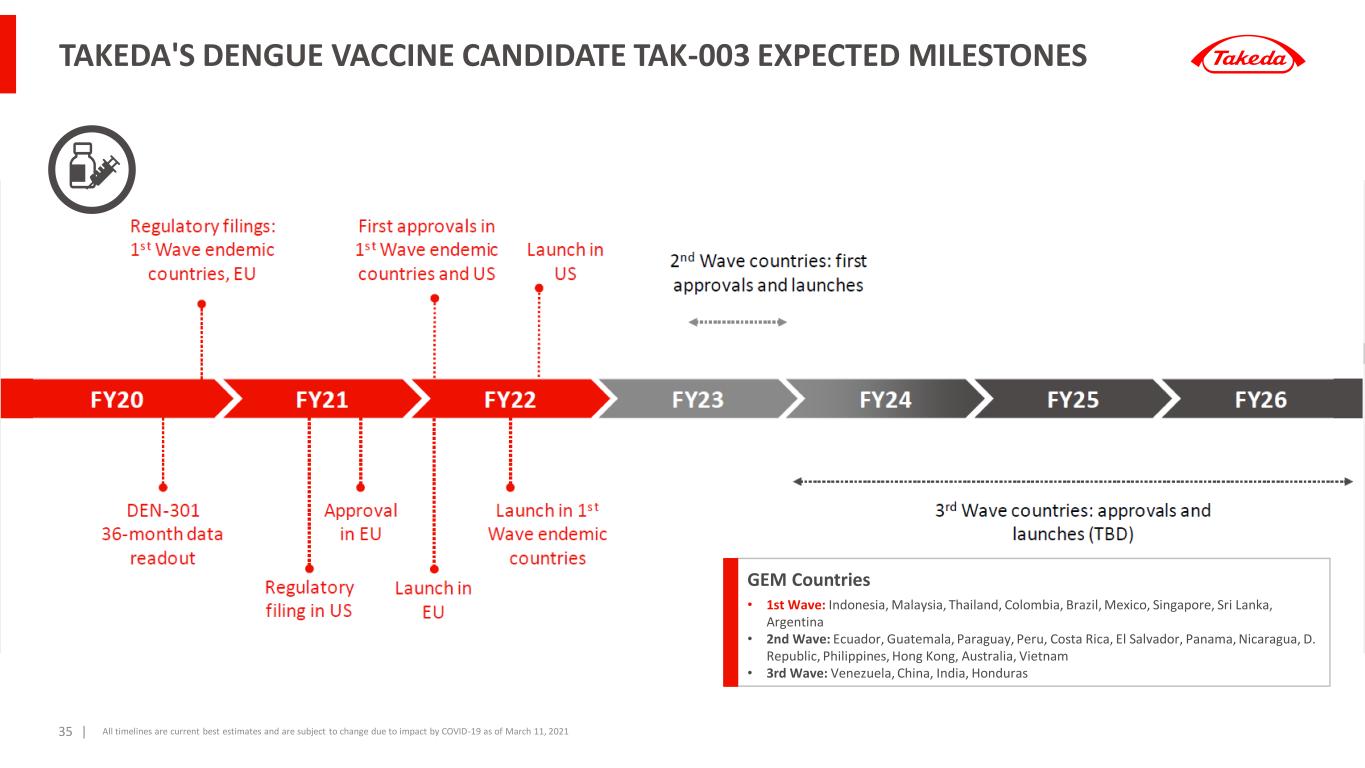
Takeda Dengue vaccine TAK-003 provides continued protection against dengue fever through 4.5 years in trial
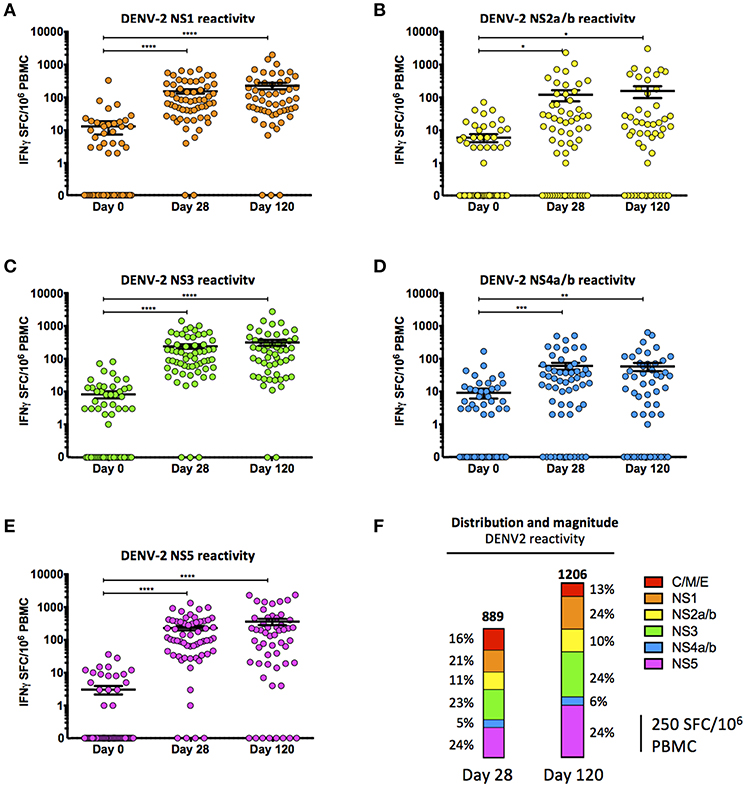
Frontiers | Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003

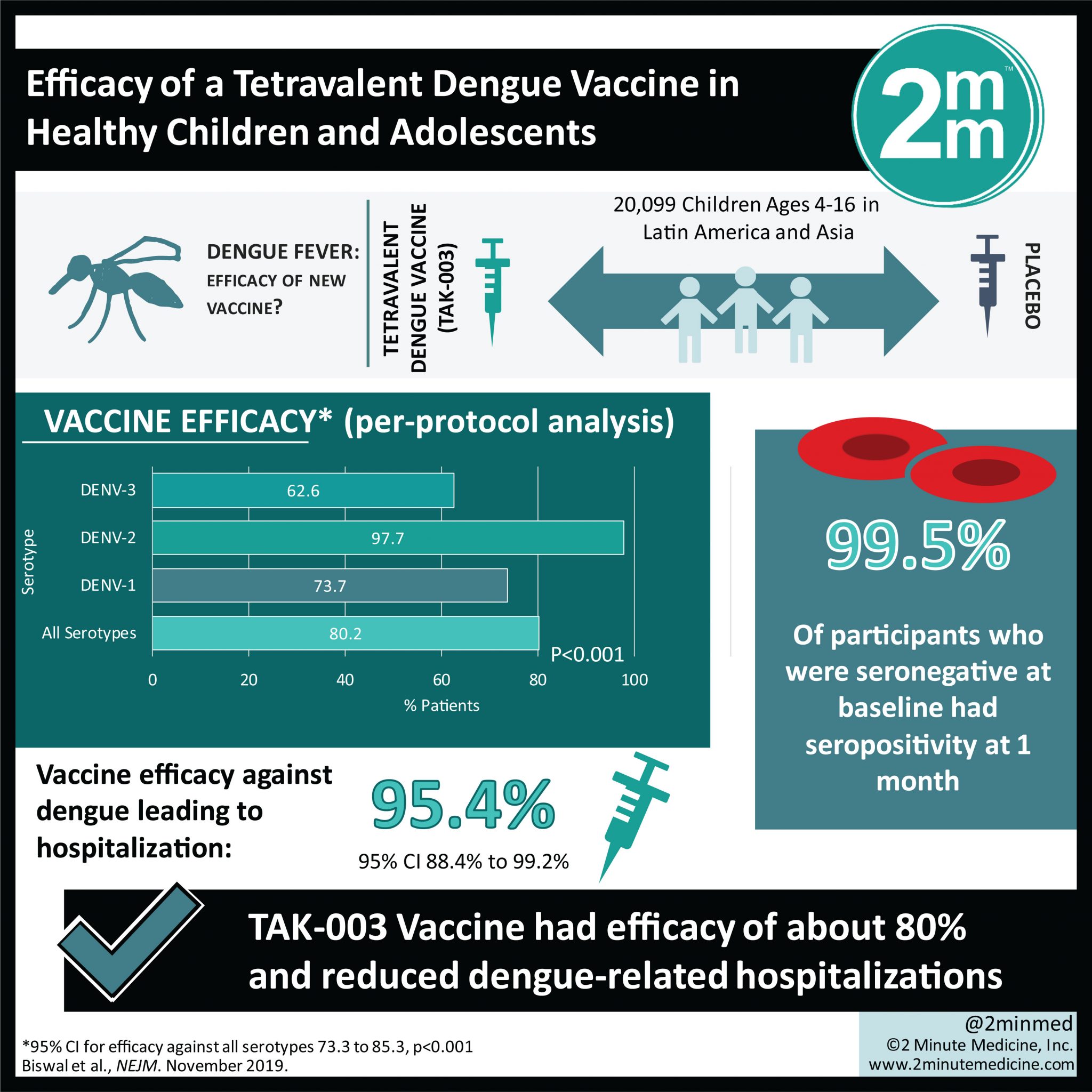
Efficacy of a tetravalent dengue vaccine in healthy children aged 4–16 years: a randomised, placebo-controlled, phase 3 trial - The Lancet

PharmaShots. - • The submission is based on a P-III TIDES trial assessing TAK-003 (0.5ml, SC) vs PBO in 20,000+ healthy children & adolescents aged 4-16yrs. to prevent dengue fever of any
Takeda's tetravalent dengue vaccine (TAK-003) receives first global approval for use in Indonesia without need for pre-vaccination testing | Asia Research News

Efficacy of a Dengue Vaccine Candidate (TAK-003) in Healthy Children and Adolescents 2 Years after Vaccination. - Abstract - Europe PMC
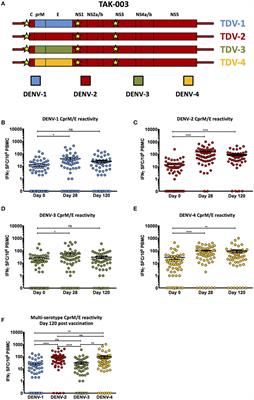
Frontiers | Assessing the Diversity and Stability of Cellular Immunity Generated in Response to the Candidate Live-Attenuated Dengue Virus Vaccine TAK-003
Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003) | PLOS Neglected Tropical Diseases

Takeda dengue vaccine TAK-003 provides continued protection against dengue fever through 4.5 years in trial
Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (TAK-003) | PLOS Neglected Tropical Diseases

Takeda's dengue shot keeps 84% of kids out of hospital regardless of prior infection, giving it a leg up over Sanofi's Dengvaxia | Fierce Biotech